Glucose Monitors Are Finally Available Over the Counter
The U.S. Food and Drug Administration (FDA) has cleared the first continuous glucose monitors (CGMs) for over-the-counter sale—a major win for health freedom and common sense.
“Giving more individuals valuable information about their health, regardless of their access to a doctor or health insurance, is an important step forward in advancing health equity for U.S. patients,” said Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, in a press release announcing the change.
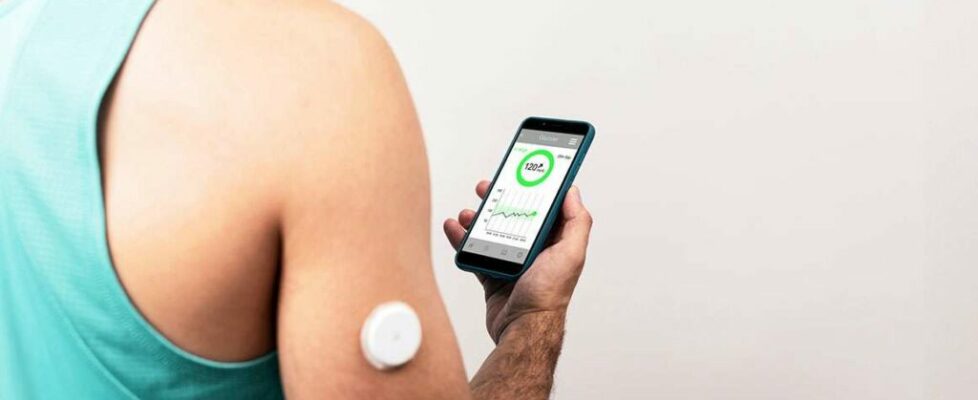
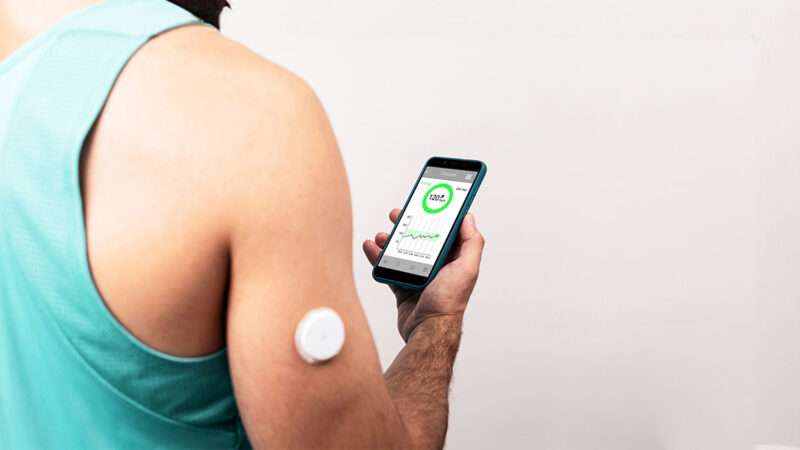
CGMs are wearable devices that continuously monitor blood sugar levels in real time. They’ve been invaluable for diabetics—but their potential goes much further. For instance, those with chronically elevated blood sugar levels, even if they don’t meet the threshold for diabetes, could use CGMs to manage their health early and avoid a future diagnosis.
Even in metabolically healthy individuals, avoiding blood sugar spikes and crashes can support weight loss, steady energy levels, and overall well-being since elevated blood sugar can cause oxidative stress and inflammation.
Before CGMs, the best way to test blood sugar levels at home was by pricking a finger and using a glucose test strip. I used this method when I was diagnosed with gestational diabetes a few years back. It was unpleasant, inconvenient—requiring lancets, test strips, and a reader—and very limited in insights, since it could only measure blood sugar levels at one discrete point in time. If my blood sugar quickly spiked beyond normal levels but also quickly fell, pricking my finger an hour or two after eating, as recommended, would fail to capture this.
CGMs offer a more convenient way of gleaning information about blood sugar responses, which can vary greatly between people or even within the same person depending on different variables. (While carbohydrates are understood to spike glucose levels higher than proteins and fats do, individual bodies can still react very differently to foods.)
Until this year, Americans needed a prescription for CGMs, which were officially approved only for use by diabetics. That meant anyone who simply wanted to learn about their own blood sugar responses either was out of luck or had to find a doctor or program willing to write an off-label prescription.
As interest in CGMs for nondiabetics grew, companies such as Levels, Signos, and ZOE emerged to cater to the market.
I signed up with Signos after learning that the “gestational diabetes” I had been diagnosed with was actually an ongoing issue with insulin production. Using the CGM that Signos provided (Dexcom’s G7 sensor) was less annoying and more informative than the finger prick method had been. But getting a CGM through Signos was not as simple—or affordable—as buying over-the-counter, as it still required an online consultation and a Signos doctor’s approval. And with the Signos program—which came with an app, community groups, weight loss tracking tools, and challenges and tests for users—I was paying for a lot of bells and whistles I really didn’t need.
For those wanting extra support, Signos and the like may be great. And it’s worth appreciating companies innovating in a heavily regulated space. But I grew increasingly annoyed that I couldn’t simply go into a drugstore and buy a CGM.
Thankfully, in March 2024, the FDA cleared Dexcom to market and granted it the legal power to sell the Stelo Glucose Biosensor System directly to consumers. It’s indicated for diabetics not using insulin and “those without diabetes who want to better understand how diet and exercise may impact blood sugar levels,” per the FDA. Abbott has also received FDA clearance to launch two over-the-counter CGMs, one (the Libre Rio) for Type 2 diabetics who don’t use insulin and one (the Lingo) for people without diabetes.
After having discontinued my CGM use for more than a year, I decided to give the Stelo a try. Getting it was simple—I just went on the Stelo website, added it to my cart, and checked out like I would with any other product. Plus, it was easier on my pocketbook.
The Signos CGM costs $449 a month, or $199 a month with a subscription. Stelo’s, on the other hand, is just $99 a month or $89 a month with a subscription. And you can get a single Lingo biosensor (lasting two weeks) for $49 or a 12-week supply for $249. We can expect CGM prices to go even lower as more over-the-counter versions are approved.
How useful CGMs are for nondiabetics is an issue still hotly debated in science and wellness communities. But a CGM is simply a device that monitors a health metric. By restricting CGMs to prescriptions and diabetics, the FDA wasn’t protecting consumers—it was keeping them in the dark.
The post Glucose Monitors Are Finally Available Over the Counter appeared first on Reason.com.